Genetic variation &
drug plasma levels
Genetic variation can impact the way our bodies metabolize medication. We’re using statistical modelling and artificial intelligence to understand the variation and predict drug exposure.
Image credit: Pawel Czerwinski
Antidepressants, liver enzymes, and treatment response
Cytochrome P450 (CYP) drug‐metabolizing enzymes regulate drug metabolism and may contribute to interindividual differences in antidepressant response. We have investigated the effects of genetic variability in CYP2C19 and CYP2D6 on plasma levels, response, and tolerability.
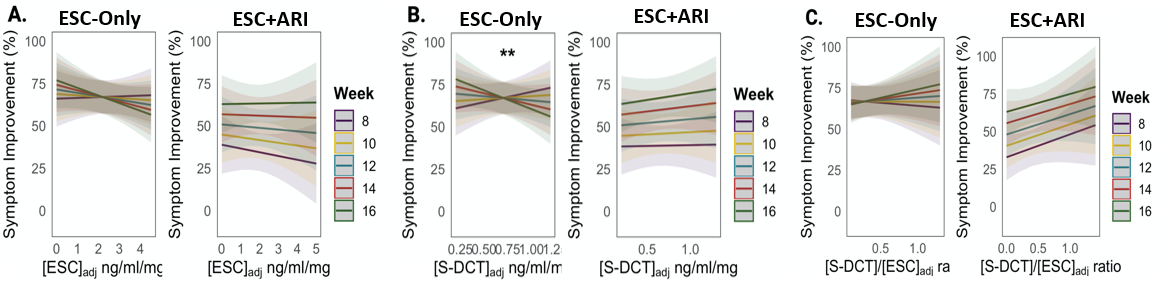
We have performed analyses on 178 individuals treated with escitalopram (ESC) from weeks 0-8 (Phase I), who at week 8, either continued on ESC if they were responders or were augmented with ARI if they were non-responders for another eight weeks (Phase II).
Results show that CYP2C19 non-NMs demonstrated 2.00% (95% CI: -3.62 to -0.37) lower symptom improvement during Phase II relative to NMs in ESC-Only, which was not observed in ESC+ARI participants. We provided evidence that this may be due to the altered pharmacokinetics of ESC with ARI co-administration, both of which compete for CYP2D6. In ESC-Only, CYP2C19 and CYP2D6 non-NMs demonstrated higher ESC concentrations at Weeks 10 and 16 compared to NMs, while ESC+ARI showed an association with only CYP2C19, but not with CYP2D6 metaboliser groups.
Instead, there was an association between CYP2D6 metaboliser groups and DHA/ARI ratio in ESC+ARI. This may be because, the competition for CYP2D6 by both ESC and ARI shifts the metabolism of ESC to be more dependent on CYP2C19, and the metabolism of ARI to be more dependent on CYP2D6 in the latter treatment arm. Our findings indicate that medication selection and dosing based on CYP2C19 genotyping could be useful to improve safety and outcome in patients on ESC monotherapy or with ARI augmentation.
This is another study where we improved upon a published population pharmacokinetic (PK) model for venlafaxine (VEN) in the treatment of depression in older adults (N=325). At both lower doses (up to 150 mg/day) and higher doses (up to 300 mg/day), CYP2D6 metabolizers impacted PK model-estimated VEN clearance, VEN exposure, and active moiety (VEN + its active metabolite O-desmethylvenlafaxine) exposure. Specifically, compared with CYP2D6 normal metabolizers, (i) CYP2D6 ultra-rapid metabolizers had higher VEN clearance; (ii) CYP2D6 intermediate metabolizers had lower VEN clearance; (iii) CYP2D6 poor metabolizers had lower VEN clearance, higher VEN exposure, and higher active moiety exposure. Overall, our study showed that including a pharmacogenetic factor in a population PK model could increase model fit, and this improved model demonstrated how CYP2D6 metabolizer status affected VEN-related PK parameters, highlighting the importance of genetic factors in personalized medicine.
Selected Publications
Men X, Taylor ZL, Marshe VS, Blumberger DM, Karp JF, Kennedy JL, Lenze EJ, Reynolds CF 3rd, Stefan C, Mulsant BH, Ramsey LB, Müller DJ. Clin Pharmacol Ther. 2024 May;115(5):1065-1074.
Effects of CYP2C19 and CYP2D6 gene variants on escitalopram and aripiprazole treatment outcome and serum levels: results from the CAN-BIND 1 study
Islam F, Marshe VS, Magarbeh L, Frey BN, Milev RV, Soares CN, Parikh SV, Placenza F, Strother SC, Hassel S, Taylor VH, Leri F, Blier P, …, Müller DJ. (2022). Translational psychiatry, 12(1), 366.