The Genomics of Antipsychotic Response
Since the first generation of antipsychotic medications was introduced in the 1950s, they have been commonly used in psychiatric treatment. From side effects to medication response, we’re looking for genetic variants to help us understand how patients respond to antipsychotics.
Impact of Clozapine on Cognition
Clozapine (CLZ) has heterogenous efficacy in improving cognitive symptoms, including working memory deficits, in patients with schizophrenia. Since, CLZ acts as an antagonist at the muscarinic M1 receptor (M1R), while N-desmethylclozapine (NDMC) is a partial agonist at M1R, the opposing effects of CLZ and NDMC may possibly contribute to the variability in CLZ’s effects on cognitive function.
Given that activation of the M1R mediates procognitive effects (Bradley et al., 2010; Melancon et al., 2013), CLZ as an antagonist at M1R is expected to worsen cognitive performance, whereas NDMC as an efficacious, potent partial agonist of M1R is expected to improve cognition. In this vein, and given the role of the cholinergic system in working memory, we and others previously showed that the ratio of CLZ/NDMC is a better predictor of working memory performance than CLZ or NDMC alone (Molins et al., 2017; Rajji et al., 2015). The smaller the ratio of CLZ/NDMC (i.e. more circulating NDMC in plasma compared to CLZ), the better the working memory performance (Rajji et al., 2015).
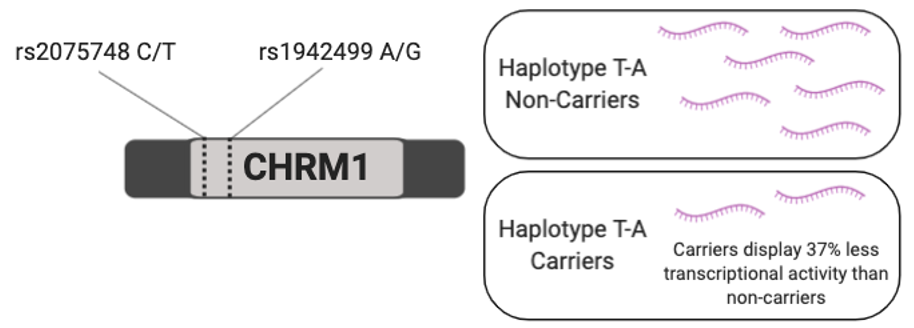
To further investigate whether the observed effects of CLZ/NDMC on working memory is mediated by M1R in patients with SCZ, we assessed the role of genetic variations in the cholinergic receptor muscarinic 1 (CHRM1) gene, which encodes M1R, in the relationship between CLZ/NDMC and working memory. Functional study results have shown that two common CHRM1 SNPs: rs1942499 and rs2075748 are associated with M1R expression (Maeda et al., 2006).
Hypothesis 1
If CLZ/NDMC ratio interacts with M1R to affect working memory performance, differences in M1R expression in haplotype T-A carriers and non-carriers is predicted to affect working memory performance.
Furthermore, CYP1A2*1F gene variant (rs762551) conferring increased enzyme inducibility to tobacco smoke and caffeine (Gunes & Dahl 2008). Since individuals with SCZ have a high prevalence of smoking compared to the general population with rates ranging from 60% to 80% (Yee et al. 2015), the presence of *1F allele and smoking behaviour may impact plasma CLZ/NDMC ratio and ultimately affect working memory performance.
Hypothesis 2
If CLZ/NDMC ratio interacts with M1R to affect working memory, interaction of CYP1A2 gene variants with smoking status is predicted to contribute to differences in plasma levels of CLZ and NDMC, and indirectly impact working memory performance.
Methods
Our sample included 30 participants meeting DSM-IV-TR criteria for SCZ or schizoaffective disorder on stable CLZ monotherapy. Participants were required to have no history of hospitalization within the past three months and needed to be on stable CLZ dosage for at least four weeks. The study protocol was approved by the CAMH Research Ethics Board and written informed consent was obtained from all participants.
Measure of Working Memory
Working memory was assessed using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) on the day blood was collected from participants.
Standardized T-scores were corrected for age and sex.
Plasma level measures:
High-performance liquid chromatography was used to measure CLZ and NDMC levels in heparinized plasma with a limit of quantitation at 100 nmol/L.
Smoking status (smoker vs. non-smoker) was determined based on the presence of cotinine levels in plasma.
Results
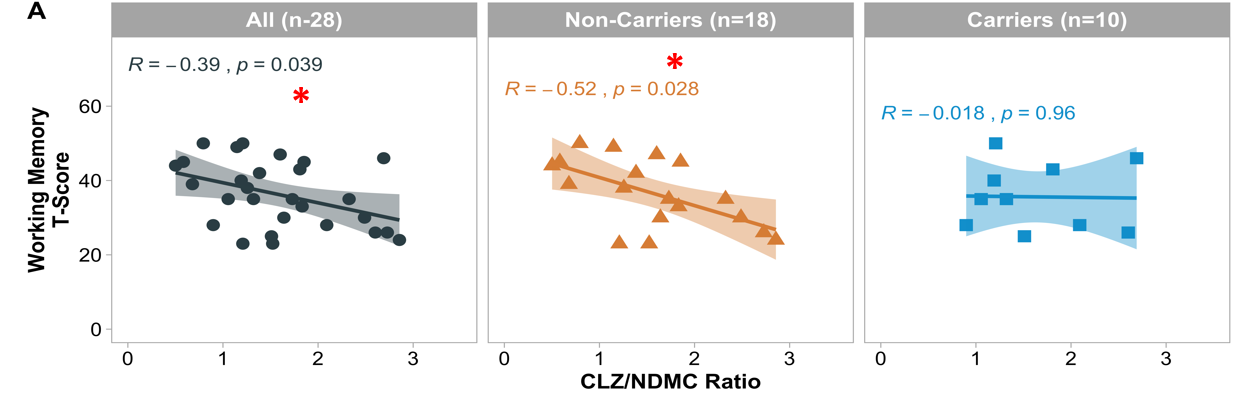
Figure 1. Spearman correlations of CLZ/NDMC ratio with working memory performance in haplotype T-A non-carriers and carriers formed by CHRM1 SNPs rs2075748 and rs1942499. *p < 0.05
Our results showed a significant negative correlation was observed between CLZ/NDMC ratio and composite working memory T-scores in the whole sample and haplotype T-A non-carriers:
Individuals with lower CLZ/NDMC ratio demonstrated better overall working memory performance.
The correlation in T-A non-carriers was stronger than the correlation observed in the whole sample (p = 0.028 versus p = 0.039).
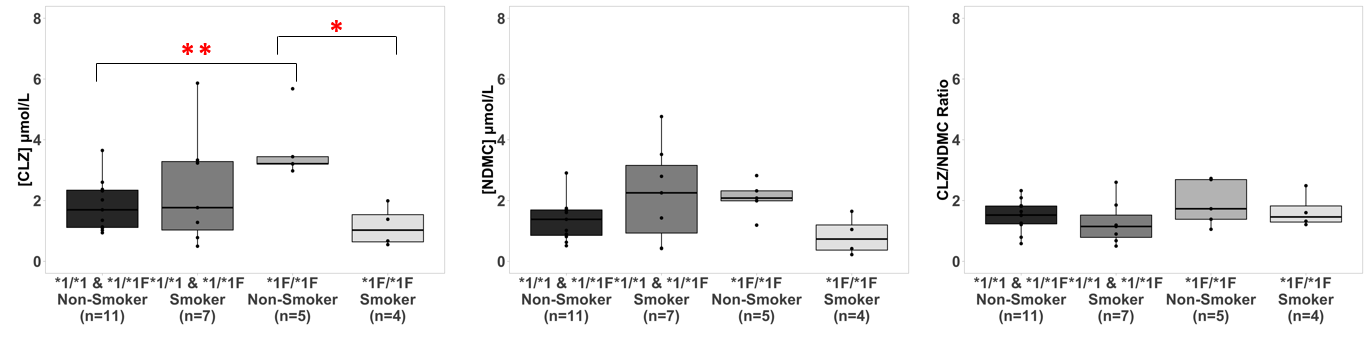
Figure 2. Association between CYP1A2 genotype and CLZ and NDMC plasma concentrations and CLZ/NDMC ratio by smoking status. * Mann Whitney U test p<0.05; ** Mann Whitney U test p<0.01
We also found significant differences in CLZ concentrations between the four groups formed by CYP1A2 genotype and smoking status (Chi square = 8.09, p = 0.044, df = 3). Smokers with *1F/*1F genotype had the lowest CLZ concentrations in plasma, which was significantly lower than *1F/*1F non-smokers (W(8) = 20.0, Z = 2.45, p = .016, CI = 1214, 5009). In contrast, non-smokers with *1F/*1F genotype had the highest CLZ concentrations in plasma compared to all other groups, with these levels being significantly higher when compared to *1/*1 and *1/*1F non-smokers (W(16) = 4.0, Z = -2.66, p = .005, CI = -3077, -836).
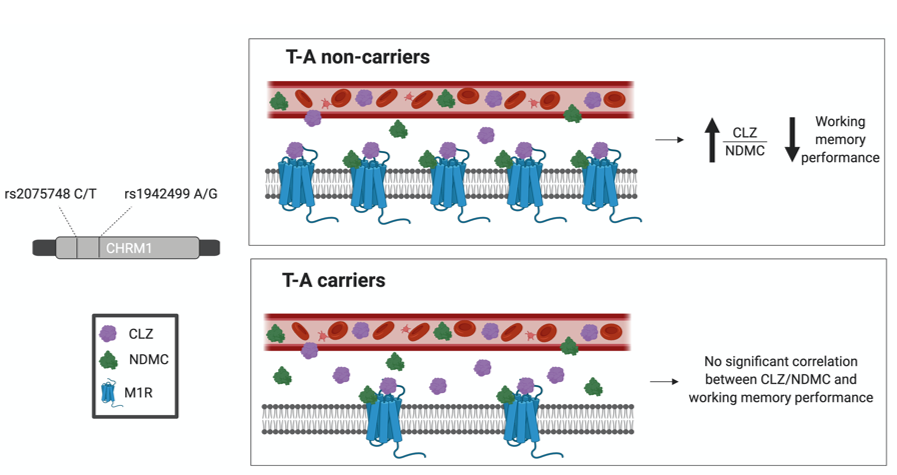
Figure 4. Hypothesized model for the effect of CHRM1 haplotype T-A (rs2075748-rs1942499) on the association between CLZ/NDMC ratio and working memory
T-A non-carriers have normal levels of transcription of the CHRM1 gene and expression of the M1R which may to greater sensitivity to variations in CLZ/NDMC ratio and consequently affecting working memory processes.
T-A carriers have lower transcription of the CHRM1 gene and expression of M1R which may eads to faster saturation of CLZ and NDMC binding sites on the M1R before it can reach clinically detectable effects of CLZ/NDMC ratio on working memory processes.
Best scenario: CHRM1 haplotype T-A non-carriers with more available NDMC (i.e. low CLZ/NDMC ratio) to bind to the M1R for the optimal benefit on working memory performance.
Take Away
Pre-emptive CHRM1 genotyping may help physicians make decisions well in advance to help mitigate potential deficits in working memory associated with CLZ treatment.
Our questions
Is the mechanism underlying clozapines’s effects on working memory performance a result of the actions of clozapine and N-desmethylclozapine at the muscarinic 1 receptor (M1R)?
Selected Publications
Contributions of cholinergic receptor muscarinic 1 and CYP1A2 gene variants on the effects of plasma ratio of clozapine/N-desmethylclozapine on working memory in schizophrenia.
Islam F, Maciukiewicz M, Freeman N, Huang E, Tiwari A, Mulsant BH, Pollock BG, Remington G, Kennedy JL, Müller DJ, Rajji TK. J Psychopharmacol. 2020 Nov 4:269881120946288.
Pharmacogenetics of Antipsychotic Drug Treatment: Update and Clinical Implications.
Yoshida K, Müller DJ. Mol Neuropsychiatry. 2020 Apr;5(Suppl 1):1-26.
Pharmacogenetics and outcome with antipsychotic drugs
Pouget JG, Shams TA, Tiwari AK, Müller DJ. Dialogues Clin Neurosci. 2014 Dec; 16(4): 555–566.